Neuroscientists have their Aplysia, geneticists have their Drosophila. We, in the field of cancer research, have HeLa – the cervical cancer cells of Henrietta Lacks, probably the most widely used cell line in the world. HeLa, and thousands of cell lines like it, form the bulk of our experimental material. Cell lines are made up of cells that have become immortalised through tumour progression, and can be cultured and passaged indefinitely. Most of them grow nicely on glass or on the coated insides of plastic culture bottles, forming a flat monolayer.
Neuroscientists have their Aplysia, geneticists have their Drosophila. We, in the field of cancer research, have HeLa – the cervical cancer cells of Henrietta Lacks, probably the most widely used cell line in the world. HeLa, and thousands of cell lines like it, form the bulk of our experimental material. Cell lines are made up of cells that have become immortalised through tumour progression, and can be cultured and passaged indefinitely. Most of them grow nicely on glass or on the coated insides of plastic culture bottles, forming a flat monolayer.
Very much of our knowledge about cancer comes from these model systems. But how well do they resemble an actual tumour?
There is a growing recognition that a tumour is a complex organ made up not only by the tumour cells themselves, but also by many kinds of supporting cells – stromal fibroblasts, blood vessel cells, etc – that enable the cancer cells to grow and proliferate. The 3D interactions between tumour cells and the extracellular matrix (that is, all the stuff that makes up tissues but lies outside of cells) are immensely important in determining the growth patterns of the cancer cells.
The tumour organ, if you wish, is possible only because of the strong self-organising properties of the diverse tissue components. Supporting cells line up next to the cancer cells. Blood vessels grow into areas that are poorly oxygenated thanks to signals that are basically the same as in healthy tissues, for example when muscles grow and need a greater blood flow.
It could be said that the tumours have subverted these normal processes for their own malignant purposes. Or, alternatively, you might argue that it is the normal tissue that drives tumour progression, leaving the cancer cell relatively innocent. (These two viewpoints appear contradictory at first, but a moment’s reflection reveals that it only seems this way because the concepts of purpose and guilt have been introduced in spite of being meaningless in this context.)
A recent paper by J Daubriac et al, in Cell Death and Differentiation, investigates a particular kind of self-organisation: when cancer cells that float freely attach to each other and start to organise into a small tissue.
A very special habitat for cancer cells, which particularly concerns mesothelioma researchers like myself, is in the fluid of the thoracic and abdominal cavities. As you might expect, there is always a small amount of fluid surrounding the lungs and the intestines, to reduce friction when they move. The surfaces are made up of a thin layer of mesothelium, which has a lubricating side facing outwards toward the opposite surface.
Occasionally, mesothelial cells come of the surface and start to live as free-floating cells in the fluid. It is thought that they can spend some time there and then settle again if they find a free spot, thereby contributing to healing any wounds in the mesothelium.
Life as a “floater” is quite different from life on the surface, because on the surface it is necessary for the cells to have polarity. Polarity is the essence of epitheliality, and means that there is a distinct surface facing downwards and to the sides (basolateral), and an apical surface with completely different characteristics facing towards the lumen, or hollow space, of whatever the epithelium in question is lining, be it an airway, a milk duct, or an intestine. The basolateral side is characterised by specialised adhesion structures; desmosomes and adherens junctions to other cells, and integrins and cadherins to the underlying connective tissue, usually a specialised basal lamina. The apical side is where secretion and absorption take place, and where specialised structures like cilia and microvilli are found.
Polarity is lost when the cell floats alone.
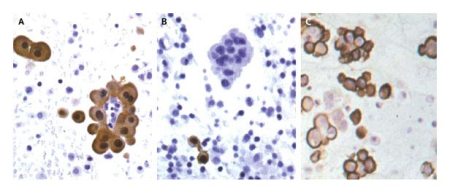
The cell must have contact with other cells in order to establish the adhesion structures that define its polarity. Normal “floaters” never achieve this. The presence in the thoracic fluid of a group of cells that adhere to each other is an ominous sign of cancer. Most cells, in fact, are programmed to commit suicide if they lose attachment, in a particular type of apoptosis termed anoikis. Cell attachment is a bit like the safety handle on a garden shredder; the moment you stop pressing it, the machine shuts off.
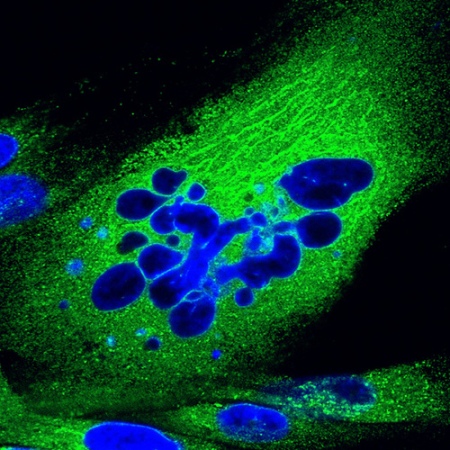
Dr Daubriac has investigated this behaviour by cultivating mesothelioma cell lines in flasks where they could not attach to the bottom. Instead, they started attaching to each other. They quickly reestablished their polarity, forming spheroids in the µm size range. Some of the cells even constructed a basal lamina in the centre of the spheroid, thus recapitulating the normal tissue features rather fully.
Two particularly interesting findings appeared when the spheroids were compared to normal monolayer culture. Firstly, their growth rate was lower. Cells in spheroids appeared to become rather content with sitting there, with neighbours on all sides, and stopped proliferating. Secondly, they were also much less likely to undergo anoikis than monolayer cells. Daubriac and his colleagues investigated the intracellular signalling pathways in some detail and were able to say that those signals that normally lead to anoikis were shut down in spheroid-growing cells.
So what is it with these tumour cells that makes them think that they have to build a new tissue? Have they acquired genetic changes that turn this program on under inappropriate conditions?
The authors’ idea is that spheroid growth is motivated by the protection against anoikis that it brings. This implies that there is a selection effect. The cells that could not form spheroids may have died and disappeared much faster, leaving the spheroids to make up a growing proportion of the floating cells. In this case, the decreased death rate may offset the decreased proliferation rate of spheroid-growing cells, leading to a net fitness gain for these cells. Furthermore, the spheroids were able to start proliferating again when they were returned to a normal culture flask and could grow out as a monolayer. It brings an image to one’s mind where spheroid cell groups are the agents of metastasis. If that is so, then a treatment directed against the spheroids might be highly valuable!
Again, we can witness the enormous explanatory potential of the evolutionary paradigm. And it always helps to keep in mind that tumours are among the most dynamic evolutionary systems that exist, because of their widespread genetic instability.
If self-organisation in spheroid growth contributes to metastasis, then it’s a bit revolutionary, because self-organisation usually works the other way! The more the cancer cells try to build a functioning tissue, the less malignant will the tumour be. Cancer cells resembling the original tissue are called well-differentiated, and then there is a whole scale with anaplastic at the bottom, which means that the cancer cells look pretty much like stem cells with no particular tissue allegiance. The differentiation state is closely liked to the patient’s prognosis.
This paper leads to a clear hypothesis that mesothelioma cells have an evolutionary fitness gain from spheroid growth. In principle, that should not be too difficult to prove or refute. I look forward to more research on the topic!
Full reference:
Daubriac, J., Fleury-Feith, J., Kheuang, L., Galipon, J., Saint-Albin, A., Renier, A., Giovannini, M., Galateau-Sallé, F., & Jaurand, M. (2009). Malignant pleural mesothelioma cells resist anoikis as quiescent pluricellular aggregates Cell Death and Differentiation DOI: 10.1038/cdd.2009.32



 Posted by evolvingideas
Posted by evolvingideas